Solvent removal can be a tricky process, especially when working with compounds sensitive to heat. Rotary evaporation offers a reliable solution by combining reduced pressure, controlled heating, and rotation to remove solvents efficiently while protecting your samples. It’s a method trusted by chemists, researchers, and manufacturers worldwide.
In this article, we’ll break down the science behind rotary evaporation, explore its applications, and share tips on choosing the right equipment for your needs. Whether you’re streamlining research or optimizing production, rotary evaporation is a technique worth mastering.
What is Rotary Evaporation?
Rotary evaporation, commonly called “rotovap,” is a laboratory technique designed for efficient solvent removal. By reducing pressure and applying gentle heat to a rotating flask, this method allows solvents to evaporate at lower temperatures, making it ideal for working with heat-sensitive compounds. It’s widely used in chemical synthesis, pharmaceuticals, and even food science for processes like concentration and purification.
The process works by lowering the boiling point of liquids through reduced pressure. A rotating flask in a heated water bath creates a thin film of liquid, increasing the evaporation area and speeding up the process. A condenser captures the vaporized solvent, which is collected separately, ensuring precision and minimal waste.
Rotary evaporation has also been leveraged for advanced crystallization processes, such as identifying new co-crystal forms of active pharmaceutical ingredients (APIs). Studies have shown that rotary evaporation accelerates crystallization kinetics, making it an indispensable tool for material development (Bag et al., 2011).
What Temperature is Used in Rotary Evaporation?
Rotary evaporation typically uses water bath temperatures between 40°C and 60°C, ensuring efficient solvent removal while protecting heat-sensitive samples. It’s vital to stay below the solvent’s boiling point to avoid overloading the condenser.
For most solvents, a temperature of 50°C works well, with adjustments made based on the solvent’s boiling point and vacuum pressure. Proper temperature control ensures efficient evaporation and preserves sample integrity.
Innovative systems, such as microcontroller-based rotary evaporators, allow for precise temperature and motor speed regulation, further optimizing performance (Shanthi & Vijayalakshmi, 2014).
What is the Principle of Rotary Evaporation?
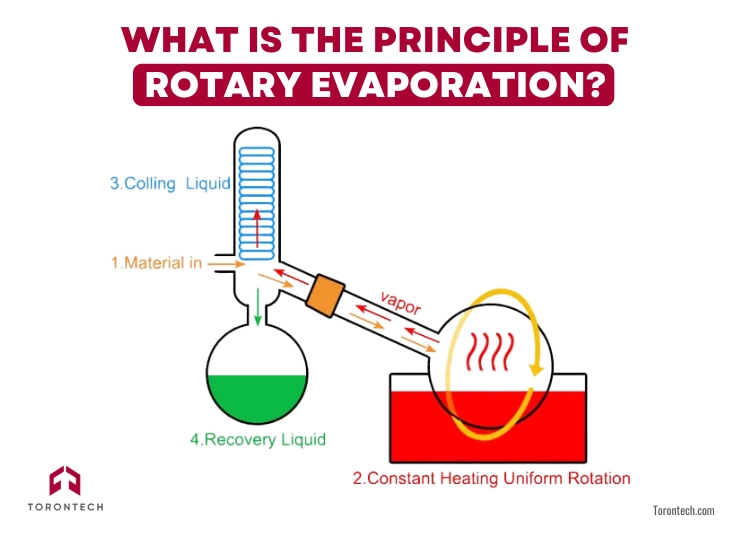
Rotary evaporation works by lowering the pressure, which reduces the boiling point of solvents, allowing them to evaporate at lower temperatures. As the flask rotates, the liquid spreads into a thin film, increasing the surface area and speeding up evaporation. This setup helps you remove solvents efficiently while protecting sensitive compounds.
A heated water bath provides just enough warmth to evaporate the solvent, while a condenser collects the vapor, turning it back into liquid in a separate flask. This ensures you recover solvents without wasting or overheating your sample.
By combining reduced pressure, rotation, and gentle heating, rotary evaporation gives you a reliable and efficient way to remove solvents, making it sustainable and efficient for diverse applications, including advanced studies in confined fluid evaporation dynamics (Gibouin et al., 2023).
What Does Rotary Evaporation Do?
Rotary evaporation is your go-to method for efficiently removing solvents from samples. It’s commonly used in tasks like concentrating solutions, purifying compounds, or recovering valuable solvents after chemical reactions. Whether you’re working in a research lab, industrial process, or even culinary science, it simplifies solvent management.
The rotating flask spreads the liquid into a thin film, accelerating evaporation while a vacuum lowers the boiling point to protect heat-sensitive compounds. The solvent vapor is then condensed and collected for reuse or disposal, making the process efficient and sustainable.
This technique saves you time, preserves the integrity of your samples, and ensures precision in applications ranging from chemical synthesis to natural product extraction. It’s a reliable solution for professionals needing quick and effective solvent removal.
Why is Rotary Evaporation Important?
Rotary evaporation is essential because it allows you to remove solvents quickly and safely without exposing sensitive samples to excessive heat. By reducing the boiling point through vacuum pressure, this method protects compounds that could degrade or react under high temperatures, ensuring the integrity of your work.
Its precision and efficiency make it a preferred tool across industries. Whether you’re concentrating a chemical solution, purifying extracts, or recycling solvents, rotary evaporation saves time and minimizes waste, all while delivering consistent results.
What is The Difference Between Rotary Evaporation and Distillation?
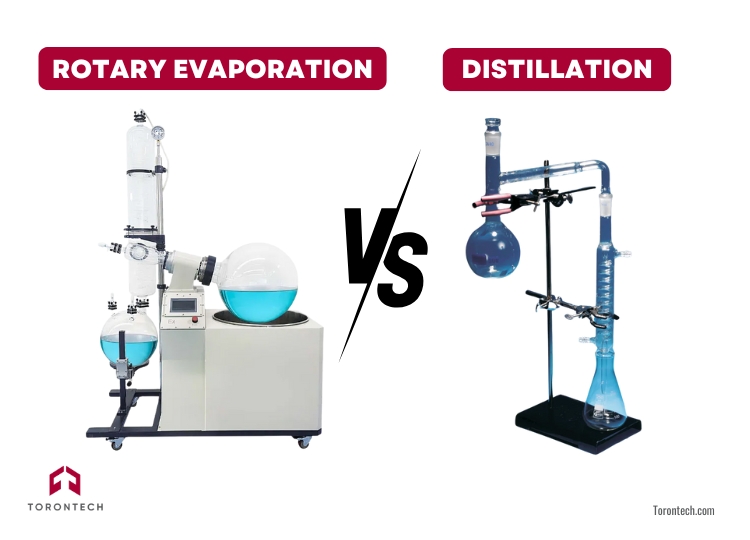
While both rotary evaporation and distillation separate substances based on boiling points, they differ in process and purpose. Rotary evaporation uses reduced pressure and gentle heat, allowing solvents to evaporate at lower temperatures.
This method is perfect for heat-sensitive compounds and small-scale solvent removal. The rotating flask creates a thin film, speeding up evaporation while minimizing the risk of bumping.
In contrast, distillation often operates at atmospheric pressure, requiring higher temperatures. It’s better suited for large-scale separations or stable compounds that can withstand heat.
Below is a comparative table summarizing the key differences:
| Aspect | Rotary Evaporation | Distillation |
| Pressure | Reduced pressure | Atmospheric pressure (or reduced in some cases) |
| Temperature | Low (protects heat-sensitive compounds) | High (suitable for heat-stable compounds) |
| Scale | Small to medium | Medium to large |
| Efficiency | Fast solvent removal | Slower, especially for complex mixtures |
| Applications | Solvent removal, concentration, purification | Separation of volatile and stable components |
| Risk of Sample Damage | Low (gentle conditions) | Higher (due to elevated temperatures) |
This table can guide you in selecting the best method based on your needs, whether you prioritize speed, scale, or compound stability.
Advantages and Disadvantages of Rotary Evaporation
Rotary evaporation offers numerous benefits, making it a staple in laboratories. However, like any technique, it has its limitations. Understanding both helps you make the most of this versatile tool.
Advantages
- Efficient Solvent Removal: Reduces boiling points through vacuum, allowing for faster evaporation.
- Protects Heat-Sensitive Compounds: Operates at lower temperatures to avoid degradation.
- Time-Saving: The thin film created by flask rotation accelerates evaporation.
- Solvent Recovery: Enables reuse of solvents, reducing waste and costs.
- User-Friendly: Modern designs with digital controls simplify operation.
Disadvantages
- Limited Scale: Best for small to medium volumes, less effective for large-scale processes.
- Risk of Bumping: Without proper precautions, sudden boiling may occur, potentially wasting samples.
- Requires Maintenance: Components like the vacuum pump and seals need regular upkeep.
- Not Suitable for All Solvents: High-boiling-point or highly viscous solvents may require alternative methods.
Despite its drawbacks, rotary evaporation remains an invaluable tool for efficient and precise solvent management. Its benefits often outweigh the limitations, especially in research and specialized applications.
Choosing the Right Rotary Evaporator Machine
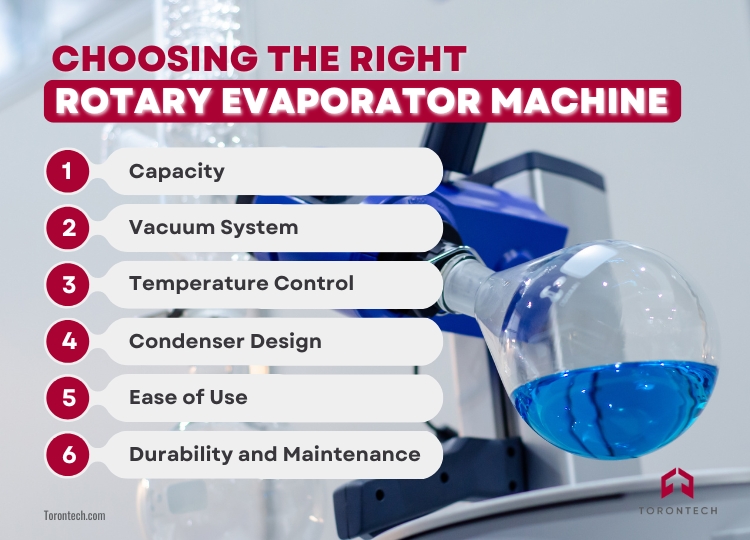
Selecting the right rotary evaporator depends on your specific needs, whether it’s for routine lab work or specialized applications. Understanding the key features and options available will help you make an informed decision. Factors to Consider:
1. Capacity
Determine the size of the flask you’ll need based on your typical sample volumes. Small-scale labs may prefer 1-5L systems, while industrial applications may require larger models up to 50L.
2. Vacuum System
Look for a robust vacuum pump with precise control to handle a range of solvents effectively. Features like automated vacuum regulation can enhance performance.
3. Temperature Control
Ensure the water bath offers accurate and adjustable temperature settings. This is vital for optimizing evaporation rates and protecting sensitive compounds.
4. Condenser Design
Choose a condenser that suits your solvent type. For highly volatile solvents, a diagonal or vertical condenser with efficient cooling is ideal.
5. Ease of Use
Digital controls, automation, and safety features like bump traps and secure clamps simplify operation and reduce the risk of errors.
6. Durability and Maintenance
Opt for high-quality materials and a design that’s easy to clean and maintain. Reliable equipment reduces downtime and long-term costs.
Closing Thoughts
Rotary evaporation is a cornerstone technique in laboratories across industries. Its ability to efficiently remove solvents, protect sensitive compounds, and deliver precise results makes it indispensable for chemists, researchers, and manufacturers alike.
Whether you’re concentrating solutions, recovering solvents, or purifying extracts, a well-chosen rotary evaporator streamlines your workflow while preserving sample integrity. By understanding the principles, advantages, and considerations of rotary evaporation, you’re equipped to harness its full potential in your work.
Investing in a quality rotary evaporator not only saves time but also enhances reliability in your processes. With the right equipment and techniques, rotary evaporation can become a seamless and valuable part of your laboratory operations.
References:
- Bag, P. P., Patni, M., & Reddy, C. M. (2011). A kinetically controlled crystallization process for identifying new co-crystal forms. CrystEngComm.
- Shanthi, J., & Vijayalakshmi, S. R. (2014). Microcontroller-based rotary evaporator for solution growth. Asian Journal of Advanced Basic Sciences.
- Gibouin, F., Nalatamby, D., Lidon, P., Medina-Gonzalez, Y. (2023). Molecular rotors for in situ viscosity mapping during evaporation of confined fluid mixtures. arXiv.