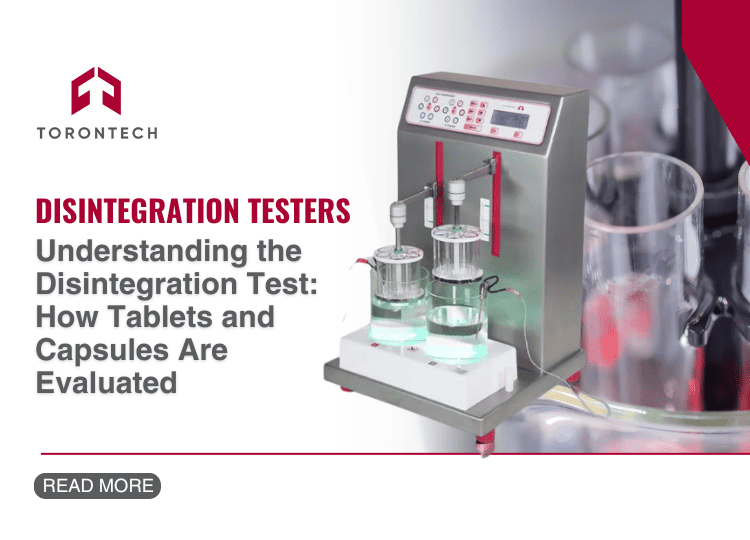
Every detail matters in pharmaceutical development. From API selection to tablet coating, each step contributes to the safety, efficacy, and reliability of the final drug product. But there’s one process that’s often underestimated in its importance: the disintegration test.
If you’re working in pharma, biotech, nutraceuticals, or even dietary supplements, understanding what a disintegration test is, how it works, and why it’s crucial can help you improve product quality, ensure compliance, and avoid costly batch failures.
In this article, we’ll walk you through the disintegration test definition, explore how the process works, compare it to dissolution testing, and show you how the right disintegration tester can streamline your lab workflows and raise your quality standards.
What Is a Disintegration Test?
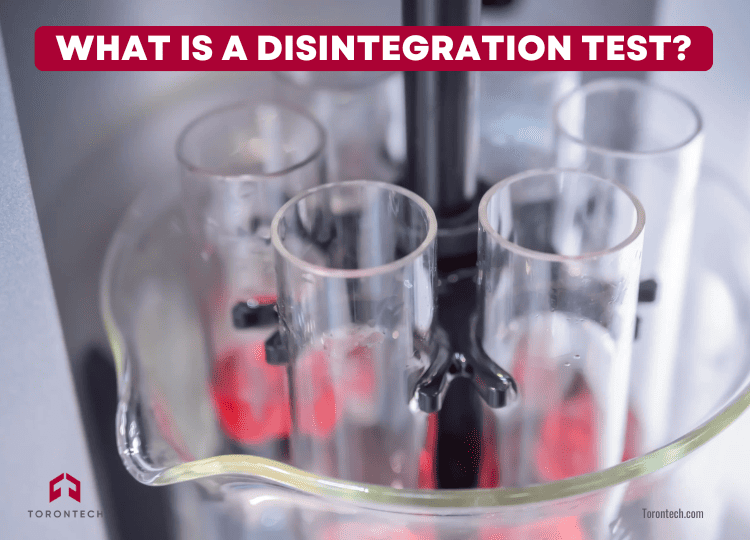
A disintegration test checks how quickly a tablet or capsule breaks down into smaller particles in a liquid medium that simulates the body’s environment—usually at body temperature (37 ± 2°C).
Why does this matter? Because disintegration is the first step toward drug release and absorption. If a tablet doesn’t disintegrate properly, the active ingredient may not be released at all, resulting in poor efficacy and potential safety concerns.
This test is especially crucial for immediate-release dosage forms, which are meant to act fast. In fact, the disintegration test USP guidelines require most tablets to disintegrate within 30 minutes.
Disintegration vs. Dissolution: What’s the Difference?
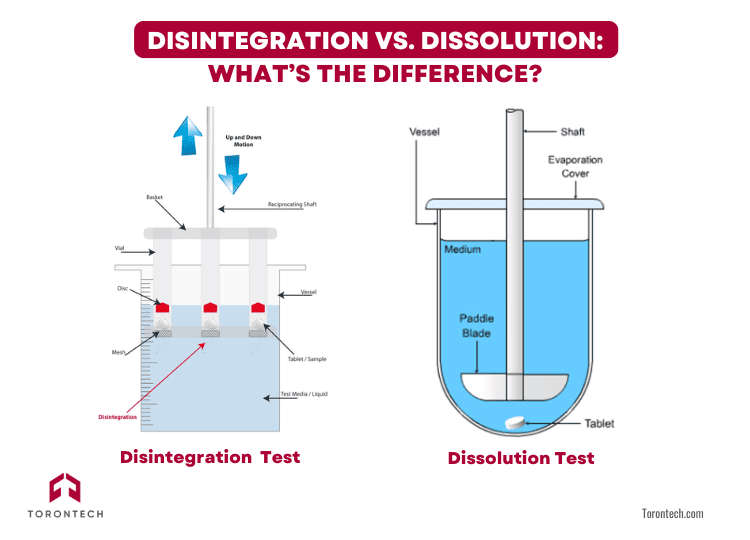
If you’re wondering about the difference between disintegration and dissolution tests, here’s a quick breakdown:
- Disintegration measures how fast a tablet or capsule breaks apart.
- Dissolution measures how fast the drug dissolves into a solution.
You can think of disintegration as the mechanical breakup, and dissolution as the chemical process that follows. Both are essential, but for some highly soluble drugs, regulatory guidelines even allow disintegration to replace dissolution testing.
That’s why many companies are exploring the benefits of disintegration and dissolution test strategies together—to better understand drug performance and streamline quality control.
Read more: Dissolution vs Disintegration: A Full Comparison
What Does a Disintegration Test Machine Do?

Disintegration test machine is a lab instrument designed to carry out the test under controlled conditions. It replicates the movement and temperature of the gastrointestinal environment to see how long a tablet or capsule takes to fall apart.
Key Disintegration Tester Parts Include:
- Basket-rack assembly: Holds up to six tubes for testing multiple samples at once.
- Glass tubes: Open-ended, fitted with wire mesh at the bottom to catch residue.
- Wire mesh screens: Typically 2.0 mm openings with 0.6 mm wire diameter.
- Heated water bath: Maintains a consistent temperature (usually 37 ± 2 °C).
- Motorized lift mechanism: Moves the basket up and down 29–32 times per minute.
- Auxiliary disks: Help keep tablets submerged, especially for coated or buoyant products.
If you’re looking for a modern and reliable option, Torontech’s Disintegration Tester is built to meet USP, EP, and JP standards. And it’s designed for ease of use, precision, and robust daily operation.
Related article: Choosing a Disintegration Tester: Automated vs Manual
Disintegration Test Procedure: Step by Step
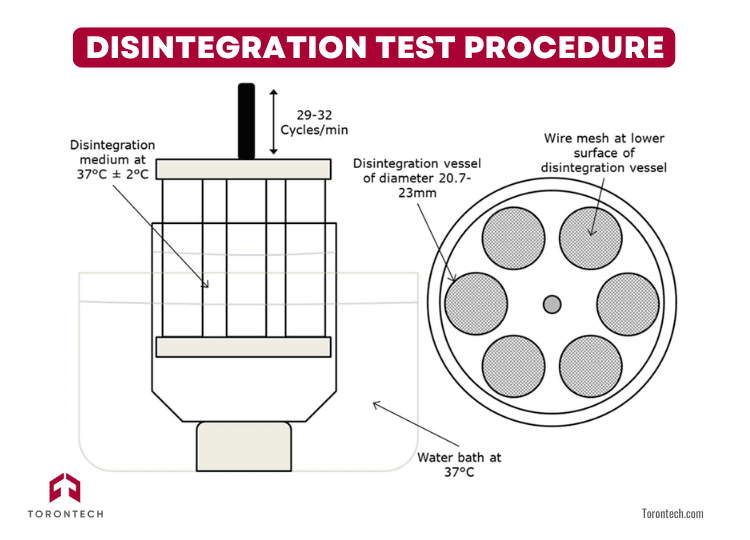
Running a disintegration test may seem straightforward, but precision and consistency are key. The process is governed by well-defined standards, such as USP <701> and USP <2040>, which outline how the test should be conducted to ensure valid and repeatable results.
Here’s a step-by-step overview of how the procedure works in practice:
1. Insert the Samples
Begin by placing one dosage unit—a tablet or capsule—into each of the six vertical glass tubes in the basket-rack assembly. These tubes are open at both ends and fitted with a fine wire mesh at the bottom to retain particles for observation.
Each sample must be handled carefully to avoid chipping or pre-test damage, which could affect the outcome.
2. Prepare the Test Medium
Fill the beaker with the appropriate immersion fluid, commonly water, simulated gastric fluid (SGF), or simulated intestinal fluid (SIF), depending on the product type. Heat the medium to 37 ± 2°C to simulate body temperature. This step is critical, as even minor temperature fluctuations can affect disintegration time.
3. Start the Test
Lower the basket-rack assembly into the beaker of heated fluid. The apparatus will then begin its motion, moving the basket up and down in a smooth, rhythmic cycle, typically 29 to 32 cycles per minute.
This motion mimics the peristaltic movements of the gastrointestinal tract, helping assess how the dosage form behaves in the body.
4. Observe and Record
Monitor the samples throughout the test duration, usually 30 minutes for uncoated tablets and up to 60 minutes for coated or enteric formulations.
The endpoint is defined as the point at which no residue of the original tablet or capsule remains, except for fragments of coating or a soft, mushy mass with no firm core.
Make sure to record:
- The total time taken for complete disintegration
- Any anomalies, such as delayed breakdown or undissolved fragments
- Whether auxiliary disks were used
5. Use Auxiliary Disks (When Needed)
For certain products, such as film-coated tablets, enteric-coated tablets, or low-density capsules that float, you’ll need to use auxiliary disks. These small, transparent plastic disks sit atop the dosage unit within each tube, ensuring the sample stays submerged throughout the test.
Without them, some formulations might not fully interact with the test fluid, leading to false failures or inconsistent results.
Disintegration Test for Tablets and Capsules
You might be wondering about the specific disintegration test for tablets and capsules. Here’s how it breaks down:
Tablets:
- Immediate-release tablets must disintegrate within 30 minutes in water or SGF.
- Coated tablets are tested with auxiliary disks and given up to 60 minutes.
- For enteric-coated tablets, there’s a two-stage test—first in acidic medium, then in buffer.
Capsules:
- Hard gelatin capsules must disintegrate within 20 minutes.
- Softgel capsules are tested using a rupture method or in water, depending on formulation.
- For some formulations, if one capsule fails, you’ll retest with an additional 6 units to verify.
The disintegration process ensures that the dosage form breaks down completely, critical for bioavailability and therapeutic effect.
Acceptance Criteria for Disintegration Test
The acceptance criteria for disintegration tests are both clear-cut and strictly enforced, because they are designed to ensure consistent product performance, regulatory compliance, and patient safety.
According to the United States Pharmacopeia (USP), here’s how the evaluation works:
- All 6 out of 6 dosage units (tablets or capsules) must fully disintegrate within the specified time frame to automatically pass.
- If 1 or 2 units fail, the test doesn’t immediately fail, but it does trigger a retest with 12 additional samples from the same batch.
- If, in total, at least 16 out of 18 units (from the original 6 + the retest group) disintegrate within the allowed time, the product still meets the disintegration test acceptance criteria.
- If 3 or more of the initial 6 fail—or if fewer than 16 of 18 disintegrate within the time limit—then the batch is considered non-compliant and requires investigation or corrective action.
These criteria apply across a wide range of oral solid dosage forms, with minor variations depending on whether the product is an immediate-release, coated, enteric-coated, or capsule formulation.
It ensures uniform performance from unit to unit and batch to batch, helping you catch formulation or process inconsistencies early. By adhering to this system, you reinforce quality assurance, strengthen consumer trust, and reduce the risk of costly product recalls or regulatory penalties.
Related article: Your Guide to USP Calibration of a Disintegration Tester
Final Thoughts
Now that you know what a disintegration test is, how it works, and why it matters—you can see just how essential it is to your pharmaceutical or nutraceutical operations.
It’s not just a regulatory box to check. It’s a vital quality control tool that protects your product, your brand, and ultimately, your patients or customers.
With a dependable disintegration tester, like the one from Torontech, you’ll have the confidence to meet standards, troubleshoot issues, and move your products forward with precision.
Frequently Asked Question (FAQ)
2. How can we improve the efficiency of disintegration testing in our high-throughput lab?
Optimizing lab workflow starts with reliable and user-friendly equipment. Modern disintegration testers often feature multiple basket assemblies, allowing simultaneous testing of different batches. For example, systems from Torontech are designed to meet USP, EP, and JP standards while emphasizing ease of use to minimize setup time and reduce the chance of procedural errors, ultimately improving overall productivity.
3. What are the operational risks if a product batch fails the disintegration test?
A failed test triggers a mandatory, resource-intensive investigation. According to USP guidelines, if one or two units fail initially, the test must be repeated on 12 additional samples. If more units fail, the entire batch may be deemed non-compliant, leading to significant financial losses from product rejection and the costs associated with corrective actions. Consistent testing with precise equipment is key to mitigating this risk.
4. When selecting a disintegration tester, what features are most important for our lab?
Look for a system that guarantees compliance and precision. Key features include a robust basket-rack assembly, precise temperature control (37 ± 2°C) of the water bath, and a consistent, smooth lift mechanism (29–32 cycles/min). To ensure long-term reliability and accurate, repeatable results, consider testers like those from Torontech, which are specifically engineered to meet these stringent pharmacopeial standards.
5. Can we use disintegration testing to replace dissolution testing and save costs?
In specific situations, yes. For certain highly soluble drugs, regulatory guidelines may permit disintegration testing to be used in place of the more complex and time-consuming dissolution testing. This can significantly streamline quality control and reduce operational costs. However, this strategy requires a thorough understanding of your drug's properties and the relevant regulatory standards to justify the substitution.