Struggling to find a reliable method for metal analysis in water, food, or pharmaceutical samples? The atomic absorption spectrophotometer might be the solution your lab needs. Trusted for decades, this instrument offers high sensitivity, straightforward operation, and proven performance in detecting trace metals at parts-per-million and even parts-per-billion levels.
In this article, you’ll discover what an atomic absorption spectrophotometer does, how it works, and why it’s still widely used across industries. From its operating principle to key components and real-world applications, this is your practical guide to mastering AAS in your lab.
Understanding the Principle of Atomic Absorption Spectroscopy
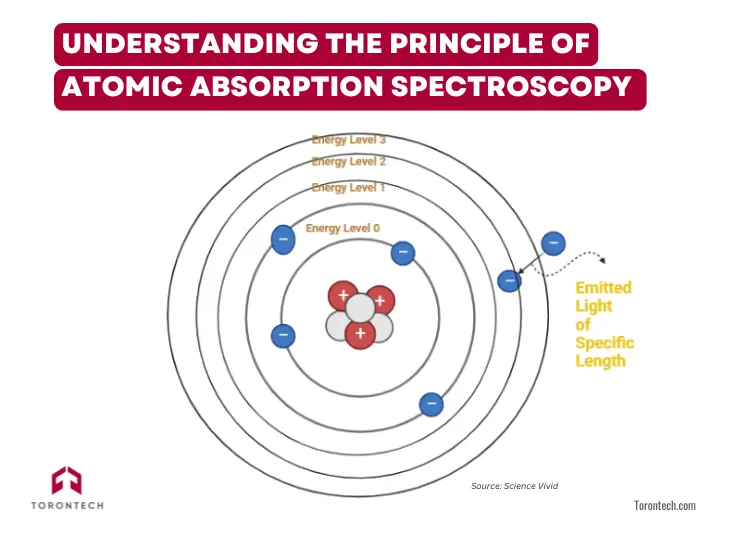
At the heart of atomic absorption spectroscopy lies a fundamental concept: atoms absorb light at specific wavelengths. When an atom is in its ground state and exposed to light at its resonant wavelength, it absorbs energy, exciting one of its electrons to a higher energy level.
This principle is governed by the Beer-Lambert Law, which states that the amount of light absorbed is proportional to the concentration of the absorbing atoms in the sample. Each element absorbs light at a unique wavelength, allowing highly selective detection.
This method is especially valuable for quantifying metals and metalloids, as these elements have sharp, well-defined absorption lines, making measurements highly specific and sensitive.
How Does an Atomic Absorption Spectrophotometer Work?
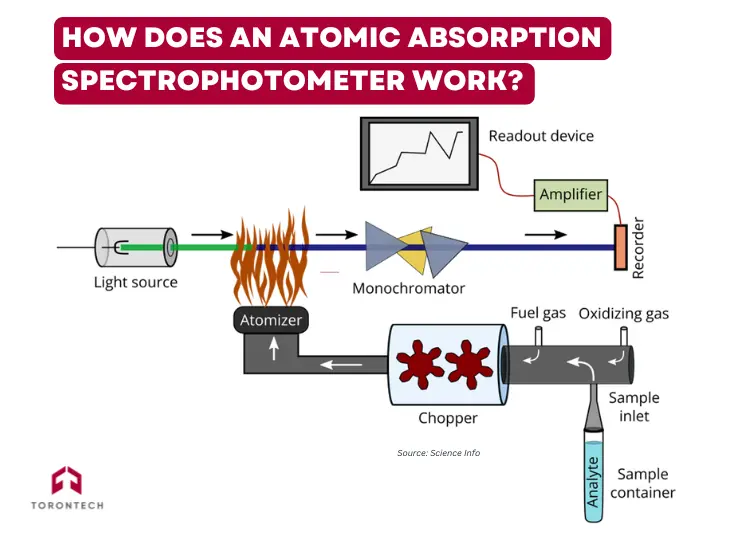
An atomic absorption spectrophotometer combines several systems into one cohesive analytical tool. Here’s how it operates:
- Sample Preparation: Liquid samples are typically used, though solids may be digested. The goal is to obtain a homogenous solution containing the target element.
- Atomization: The sample is introduced into an atomizer (either a flame or a graphite furnace), which breaks it down into free ground-state atoms.
- Light Emission: A hollow cathode lamp (HCL) emits light specific to the target element.
- Light Absorption: As the beam passes through the cloud of atoms, some of the light is absorbed.
- Wavelength Selection: A monochromator isolates the desired wavelength to minimize interference.
- Detection: A photomultiplier tube (PMT) or CCD detector measures the drop in light intensity.
- Data Processing: A computer compares the reading to a calibration curve to determine the element’s concentration.
This step-by-step process allows AAS instruments to deliver precise quantitative analysis — often at trace levels.
What Elements Can Be Analyzed?
AAS is primarily designed to detect metals and metalloids, including:
- Lead (Pb)
- Cadmium (Cd)
- Zinc (Zn)
- Copper (Cu)
- Arsenic (As)
- Iron (Fe)
- Mercury (Hg)
- Nickel (Ni)
- Manganese (Mn)
- Selenium (Se)
- Bismuth (Bi)
These elements are often measured in concentrations as low as parts per billion (ppb) using graphite furnace AAS (GFAAS), or parts per million (ppm) using flame AAS (FAAS).
Key Components of an Atomic Absorption Spectrophotometer
Each AAS system includes several critical components:
- Light Source: Usually a hollow cathode lamp (HCL) containing the element to be measured. Deuterium lamps are used for background correction in the UV range.
- Atomizer:
- Flame atomizers (using air-acetylene or nitrous oxide-acetylene flames) are best for routine analyses.
- Graphite furnaces offer higher sensitivity and lower detection limits.
- Monochromator: Filters out unwanted wavelengths, focusing only on the absorption line of the target element.
- Detector: Converts light into an electrical signal. Photomultiplier tubes (PMT) are standard, though CCD detectors are now used in high-resolution systems.
- Computer & Software: Controls parameters, builds calibration curves, and processes results in real-time.
What Types of Samples Can Be Analyzed?
AAS works best with samples in liquid form. These can include:
- Water (drinking, wastewater, environmental)\
- Digested solids (soils, rocks, food, metals)
- Biological fluids (blood, urine, plasma)
- Industrial materials (plating baths, alloys, catalysts)
Sample preparation may involve acid digestion, filtration, and dilution to ensure homogeneity and compatibility with the atomization system.
Industries That Rely on Atomic Absorption Spectrophotometers
AAS is used in a wide range of industries and applications:
- Environmental Monitoring: Lead in drinking water, mercury in soil, arsenic in groundwater
- Food and Beverage: Trace metal screening for safety and labeling compliance
- Pharmaceuticals: Residual metal analysis as required by USP <232>/<233>
- Mining and Metallurgy: Quantifying valuable or toxic metals in ores and smelting products
- Clinical and Biomedical: Assessing nutritional deficiencies and toxic exposure
- Agriculture and Cannabis: Fertilizer formulation and heavy metal screening
AAS is often cited in regulatory standards from the EPA, FDA, WHO, and ISO — making it a trusted compliance tool.
How Sensitive Is AAS for Trace Metal Detection?
AAS is very sensitive, especially when using graphite furnace atomization or hydride generation techniques. You can achieve:
- Low ppm detection with flame AAS
- Sub-ppb detection with graphite furnace AAS
- High selectivity, as each element absorbs light at its own wavelength
While not as sensitive as ICP-MS, AAS remains effective for most regulatory and research applications, especially where cost and simplicity matter.
Light Sources Used in AAS
Most AAS systems use:
- Hollow Cathode Lamps (HCL): Emit narrow spectral lines for element-specific detection
- Deuterium Lamps: Used for background correction
- Continuum Sources (e.g., xenon arc lamps in HR-CS AAS): Offer broader wavelengths and enable simultaneous analysis
Older systems rely solely on HCLs, while newer ones use high-resolution continuous-source AAS for faster, more flexible workflows.
Is AAS Suitable for Regulatory Compliance?
Yes and it’s widely recognized in national and international standards. AAS is used to meet requirements from:
- EPA Method 200.9 (metals in water)
- USP <232>/<233> (elemental impurities in drugs)
- AOAC methods for food safety
- ISO 15586 (water analysis by GFAAS)
A properly calibrated AAS instrument with routine validation and background correction is fully capable of regulatory-grade accuracy.
Pros and Cons of Using Atomic Absorption Spectrophotometer
AAS remains a reliable choice for labs focused on elemental analysis. They offer notable benefits in sensitivity and specificity, but they also come with limitations that may impact throughput, flexibility, or usability in some settings.
A. Advantages of Atomic Absorption Spectrophotometer
- High Accuracy and Sensitivity
AAS delivers precise results with error rates typically between 0.5–5%, and it can detect elements down to ppb levels, especially with graphite furnace atomization.
- Cost-Effective for Routine Analysis
While initial investment may be moderate to high, the cost per test is low, and consumables are inexpensive compared to techniques like ICP-MS.
- Wide Dynamic Range
AAS can measure both trace and high concentrations, reducing the need for multiple instruments or dilution steps.
- Versatility Across Sample Types and Industries
Suitable for analyzing liquids, digested solids, and gases, AAS is used in environmental labs, clinical research, food safety, mining, and more.
- Compact and User-Friendly
Many systems have a small footprint, with easy-to-use software and high-throughput capability via autosamplers, making them ideal for space- and time-efficient labs.
B. Disadvantages of Atomic Absorption Spectrophotometer
- Limited to Metal and Metalloid Analysis
AAS cannot detect non-metals or organic compounds, restricting its use to elemental testing. - Single-Element Analysis at a Time
Each element requires a specific hollow cathode lamp, and the system measures only one element per run, limiting efficiency in multi-element workflows. - Sample Preparation Requirements
Most samples must be converted into liquid form, often requiring acid digestion, which can be time-consuming and introduces the risk of contamination. - Potential Interference and Background Effects
Matrix, chemical, or spectral interferences can affect results, particularly in complex or dirty samples, requiring careful correction techniques. - Use of Flammable Gases (for Flame AAS)
Acetylene and nitrous oxide flames present safety and environmental concerns, demanding proper ventilation and gas-handling protocols.
Common Troubleshooting Issues with AAS
Even experienced users occasionally face instrument issues. Here are a few examples:
- Unstable readings: May result from lamp flicker, dirty optics, or incorrect burner alignment.
- Low sensitivity: Often caused by clogged nebulizers, degraded lamps, or poor sample prep.
- Interference from the matrix: Use background correction techniques or matrix modifiers.
- Calibration drift: Make sure to use fresh standards and run blank samples routinely.
Modern AAS systems often include automated alerts, diagnostics, and safety interlocks to improve reliability and user safety.
Final Thoughts: Why AAS Still Matters in Modern Labs
Atomic absorption spectrophotometers offer a unique blend of simplicity, specificity, and affordability for elemental analysis. While newer techniques like ICP-OES and ICP-MS offer multi-element detection and ultra-low detection limits, AAS still shines when:
- You need to analyze one or a few elements.
- You’re working with limited budgets or space.
- You require regulatory-compliant trace metal testing without the complexity of plasma-based systems.
From environmental labs to pharmaceutical QC, AAS remains a dependable workhorse — one that’s well worth understanding, maintaining, and using effectively.








